Congratulations on Completing Trimester 1 of Chemistry!
|
Week 12 Resources/ Assets |
|
Updated November 24, 2018
Please make sure you READ everything on this page or you will miss important details. I will archive past weeks Announcements pages for a limited time only under the Trimester 1 and 2 tabs, if you need to go back to a particular weeks information for any reason. Week 11 in Review (Before Thanksgiving Break)The Tuesday before Thanksgiving Break we completed Chapter 7 - Chemical Quantities. All of you collected the data to complete the Lab: Measuring Mass as a Means of Counting. Please make sure you complete all the calculations on the lab, and return on Tuesday.
Recollect that we learned how to calculate the percent composition by mass of an element within a compound. As consumers, we are often concerned with the percent composition of matter whenever using and purchasing fertilizers, when purchasing jewelry (is the ring 10K or 14K gold?), and of course, chocolate. To refresh your memory, consider the example of a calculation involving percent composition, below, that comes from your homework. Calculate the percent composition by mass when 29.0 g Ag combines completely with 4.30 g S to form a compound:
We also spent some time learning how to calculate the empirical formula of a compound. Consider the steps to this by examining Practice Problem 35(a): Calculate the empirical formula of a compound that is 94.1% O, and 5.9% H.
(5.9 g H) (1 mol H/1.01 g H)= 5.84 mol H;
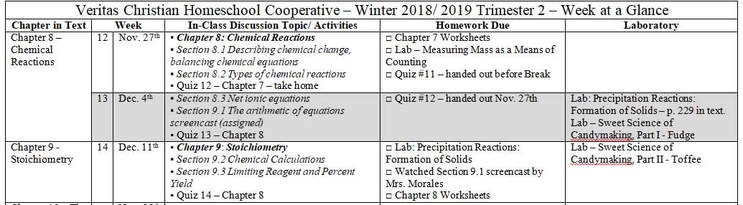
Looking Ahead to Week 12 - Return from Thanksgiving Break When we return from break, we will start Chapter 8 - Chemical Reactions. Chapter 8 is divided accordingly: Section 8.1 - Describing Chemical Change Section 8.2 - Types of Chemical Reactions Section 8.3 - Reactions in Aqueous Solution The five major classes of chemical reactions are: 1. Combination (synthesis) reactions 2. Decomposition Reactions 3. Single-Replacement Reactions 4. Double-Replacement Reactions 5. Combustion Reactions Chapter 8 is not quite as math-intense as Chapter 7, and I believe you will find it much more interesting, as we learn about these different types of chemical reactions. The two videos below will provide for you a good introduction to Chapter 8. Trimester 2 Schedule
Below I have included an agenda for classes up through the Christmas Break. When I queried the class concerning the preference for laboratory time in class versus discussion - the class overwhelmingly desired laboratory time (yay!). Then, when you were all asked whether you would find food labs more interesting than traditional chemistry labs, again your preference for food labs was nearly unanimous. Note there are two food labs coming up in December, along with a traditional chemistry lab.
|
So that you are adequately prepared for classes next week, please complete the following:
Tuesday, November 27th:
|