"An experiment is a question which science poses to Nature, and a measurement is the recording of Nature's answer." - Maxwell PlankEvery time you make a measurement, you must assign not only a correct numerical value, but a unit to the measurement as well. Without the units, it is impossible to understand the measurement completely. Imagine if you were telling a friend where your house is in relation to theirs: you could not say, "My house is sixteen." Are you sixteen kilometers from your friend or sixteen miles?
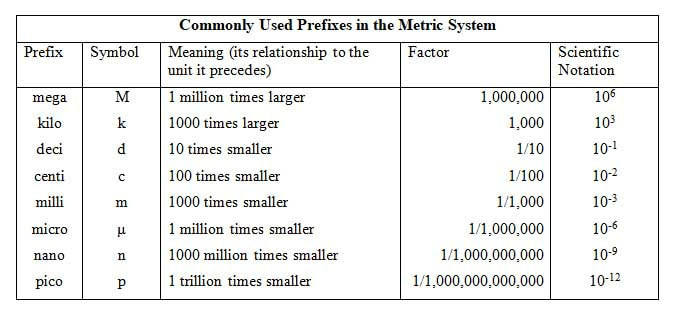
All measurements depend upon units that serve as reference standards. Those typically used in the science disciplines are those of the metric system. The metric system, while it may be unfamiliar to you, is preferred due to its simplicity, ease of use, and standardization. Think of this: if there are twelve inches to a foot, and three feet to a yard, could you convert your height in inches to yards quickly? You would have to go through a few conversion factors that are based on 12:1 and 3:1. All metric units are based on 10 or multiples of 10, which makes conversions between units easy.
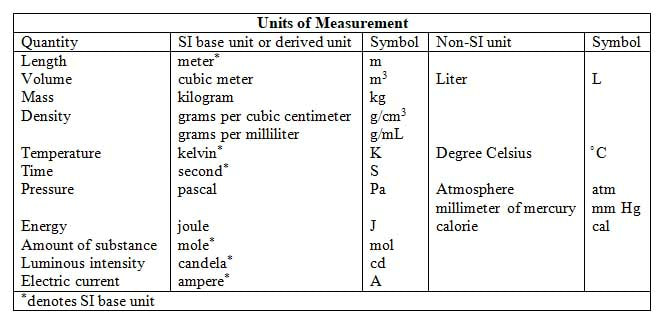
The table below shows some units of measurement. Notice that from only a few base units (i.e., kilogram, second, meter, joule) all other units are derived (density, volume, pressure). While all measured quantities can be reported using SI units, sometimes non-SI units are used for convenience, historical reasons, or practical purposes. The most commonly used measurements involve length, mass, and volume.
Units of LengthThe SI unit of length is the meter (m); all measurements of length (as discussed in Module 4) can be expressed in meters, whether we are talking about the radius of an atom or the distance of the universe. For large distances, measurements are most appropriately expressed in kilometers (km). The prefix kilo- means 1000, making 1 km equal to 1000 m. As a matter of reference, standard marathon distance races are about 42,000 m, but more conveniently expressed as 42 km.
For very tiny distances - such as that between two atomic nuclei, the prefix nano- (1 nanometer equals 0.0000000001 m), micro- (1 micrometer equals 0.000001 m) is often used. We are more familiar with centimeters (1 centimeter equals 0.01 m) or even millimeters (1 millimeter equals 0.001 m). The table below shows some of the more commonly used prefixes. Units of VolumeThe degree to which an object occupies space in all three dimensions is characterized by volume. It is easy to calculate the volume of any cubic or rectangular solid by multiplying its length by its width by its height. Since all this multiplication takes place, volume is said to be a derived unit. The SI unit for volume is the cubic meter.
In everyday use, we are more familiar with the non-SI unit for volume, the liter. This unit is more convenient for measuring liquids directly, and irregular solids by volumetric displacement (which you will learn about in your chemistry class). Units of MassNot to be confused with weight, mass is a measurement of the quantity of matter an object has, whereas weight measures the force on an object due to its mass and the gravitational attraction of Earth. To tease these two definitions apart, an astronaut's weight on the moon would be only one sixth of that on Earth, which accounts for his ability to jump much higher on the moon.
The SI unit for mass is that of the kilogram (kg). To give you an idea as to its mass, one kilogram was originally defined as the mass of 1 liter of liquid water at 4 degrees Celsius. |
Module 5 ResourcesCheck-off List of Things to Do:
|